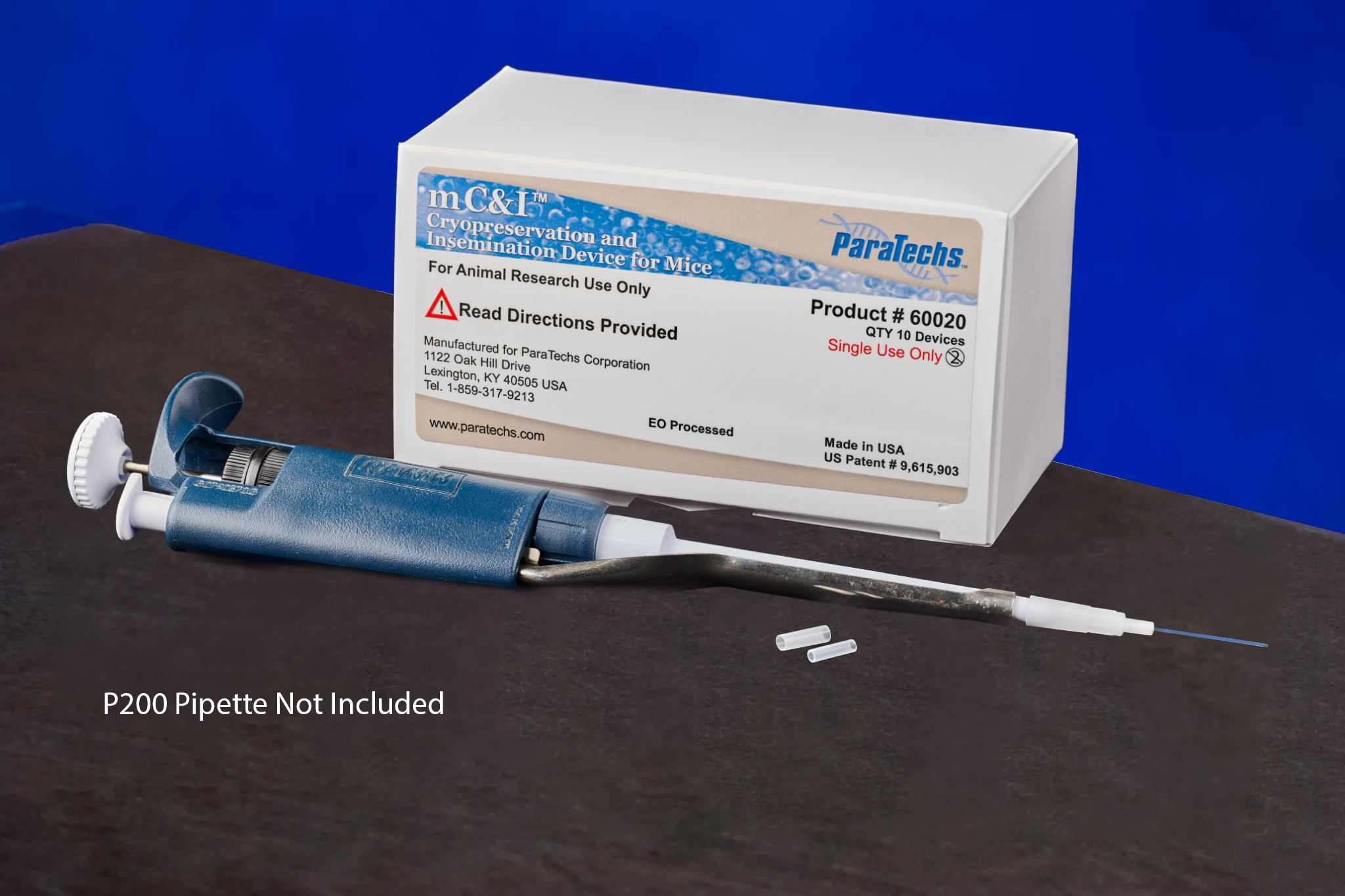
mC&I Device for Mice 60020 (10 Devices per Box)
60020 (10 devices per box)
$309.00
10 Devices/Box
ParaTechs’ mC&I Device for Mice 60020 is designed for assisting with non-surgical mouse artificial insemination and uterine physiological study procedures. The device allows uterine delivery of up to 40µl of liquid using a standard 200µl pipette. The mC&I device eliminates the need for anesthesia, analgesia, and post-operative recovery normally required for conventional procedures. Compare this methodology with the time, expense, and training required to accomplish the same task with surgical methods and you will discover that the procedure is a rapid and cost-effective refinement in alignment with the 3Rs principles of humane experimental techniques as developed by W.M.S. Russel and R.L. Burch. Specific protocols for Chlamydia research are available through ParaTechs upon request (info@paratechs.com).
The mC&I Device for Mice 60020 is manufactured in the USA, solely for ParaTechs Corporation, by an FDA Registered Medical Device Manufacturer and ISO 13485:2003 Registered Company and is EtO (Ethylene Oxide) sterilization processed. Patent Information: Non-Surgical Embryo Transfer Method and Apparatus, United States Patent 9,615,903. [PDF]

10% Discount on orders of 10 or more boxes. Discount applies automatically at checkout.
For international customers, please contact ParaTechs for orders over 8 boxes. This website is currently unable to process the Customs Documents for orders over $2500.
Uses
Mouse Sperm Transfer for Artificial Insemination (AI):
- Fresh sperm
Pathogen Transfer:
- Chlamydia trachomatis
Material Transfer:
- Decidualization procedures
- Pharmaceuticals
Advantages
- 3Rs compliant
- Eliminates the pain and distress of surgery
- No anesthesia or analgesia required
- Eliminates need for post-surgical monitoring of animals
- Reduces regulatory burden by eliminating need to justify survival surgery
- Eliminates surgical instruments and time-consuming pre- and post-surgical processes
- Greatly reduces training time required to become proficient
- Reduces costs of procedures vs surgical alternatives
- Rapid procedure
- Convenient
- Sterile and ready to use
Documents
mC&I Instructions for artificial insemination
Videos
Watch ParaTechs' JoVE article "Inducing Pseudopregnancy in Female Mice Without the Need for Vasectomized Males Prior to Non-Surgical Embryo Transfer or Artificial Insemination"
- Author Spotlight
- Cervical Manipulation Procedure for Use with Artificial Insemination in CD1 Mice (CM Rod 90050) (mC&I Device 60020)
- Non-Surgical Embryo Transfer Procedure for Use with Cervical Manipulation in CD1 Mice (CM Rod 90050) (mNSET Device 60010)
Inducing Pseudopregnancy in Female Mice Without the Need for Vasectomized Males Prior to Non-Surgical Embryo Transfer or Artificial Insemination JoVE Published: July 7, 2023